Introduction
Methyl dihydrojasmonate: is an aroma compound that smells similar to jasmine. In racemic mixtures the odor is floral and citrus while epimerized mixtures exhibit a dense buttery-floral odor with odor recognition thresholds of 15 parts per billion.[1]The compound is also known as hedione or kharismal. Its boiling point is 110°C at 0.2 mmHg and it has an refractive Index: 1.45800 to 1.46200 (20.00°C).
Methyl Dihydrojasmonate (MDJ) is jasmone-like compound that appears colorless to pale yellow transparent oily liquid with floral and jasmine-like odor.
It is extracted from natural oil with floral, jasmine, citrus freshness.Methyl Dihydrojasmonate is industrially prepared by the condensation of 2-pentylcyclopent-2-en-1-one and diethyl malonate at the presence of catalyst, and then through the hydrolysis, decarboxylation, and esterification reactions at 160-180℃.
Methyl Dihydrojasmonate is a very important fragrance in modern perfume industry and commonly used in the preparation of oriental perfumes.Methyl Dihydrojasmonate was developed by Demole and Lederer In 1960s, and produced by Firmenich. (Germany Patent: 1150483, Helv. chim. acta, 1962, 45’2′, 685)
1. WHAT IS HEDIONE?
Not commercial name: Methyl Dihydrojasmonate
This ester, only ‘recently’ brought into the market as a commercially available perfume chemical, is intended for use in artificial Jasmin absolute, Jasmin and Tuberose bases, and as a trace additive in powerfully floral fragrances.
It serves as an economical substitute for Methyl jasmonate (post coming) but does not have the overwhelming sweetness and diffusive power of that material. In 1969, the title ester cost nearly twice as much as Jasmin absolute from Italian concrete.
Methyl dihydro jasmonate is said to smell less intensive as its purity increases. When you have perceived this substance once, you have the impression of blossoming flowers everywhere in nature, especially in springtime. The sensation is a kind of radiation, which conjures up the picture of a sunbeam sizzling your nose into a springtime feel.
The compound that without a doubt has most influenced modern perfumery and has allowed great artists to develop their ideas with inspiration. It was used for the first time in Eau Sauvage and in Diorissimo, and it has become famous because it gives compositions a delicate, fresh, smooth, radiant, warm, elegant character that blends well with all kinds of perfumes from floral-citrus to woody, chypre and oriental.
2. Fragrance material review on methyl dihydrojasmonate
Author links open overlay panelJ. Scognamiglio a, L. Jones b, C.S. Letizia a, A.M. Api a
1. Abstract
A toxicologic and dermatologic review of methyl dihydrojasmonate when used as a fragrance ingredient is presented. Methyl dihydrojasmonate is a member of the fragrance structural group ketones cyclopentanones and cyclopentenones. The common characteristic structural element of the group members is a cyclopentanone or cyclopentenone ring with a straight or branched chain alkane or alkene substituent. This review contains a detailed summary of all available toxicology and dermatology papers that are related to this individual fragrance ingredient and is not intended as a stand-alone document.
Available data for methyl dihydrojasmonate were evaluated then summarized and includes physical properties, acute toxicity, skin irritation, mucous membrane (eye) irritation, skin sensitization, elicitation, phototoxicity, photoallergy, toxicokinetics, repeated dose, reproductive toxicity, and genotoxicity data.
A safety assessment of the entire ketones cyclopentanones and cyclopentenones will be published simultaneously with this document; please refer to Belsito et al. (this issue) for an overall assessment of the safe use of this material.
Belsito, D., Bickers, D., Bruze, M., Calow, P., Dagli, M., Fryer, A.D., Greim, H., Hanifin, J.H., Miyachi, Y., Saurat, J.H., Sipes, I.G.,. A toxicologic and dermatologic assessment of ketones cyclopentanones and cyclopentenones when used as fragrance ingredients. Food and Chemical Toxicology.
2. Introduction
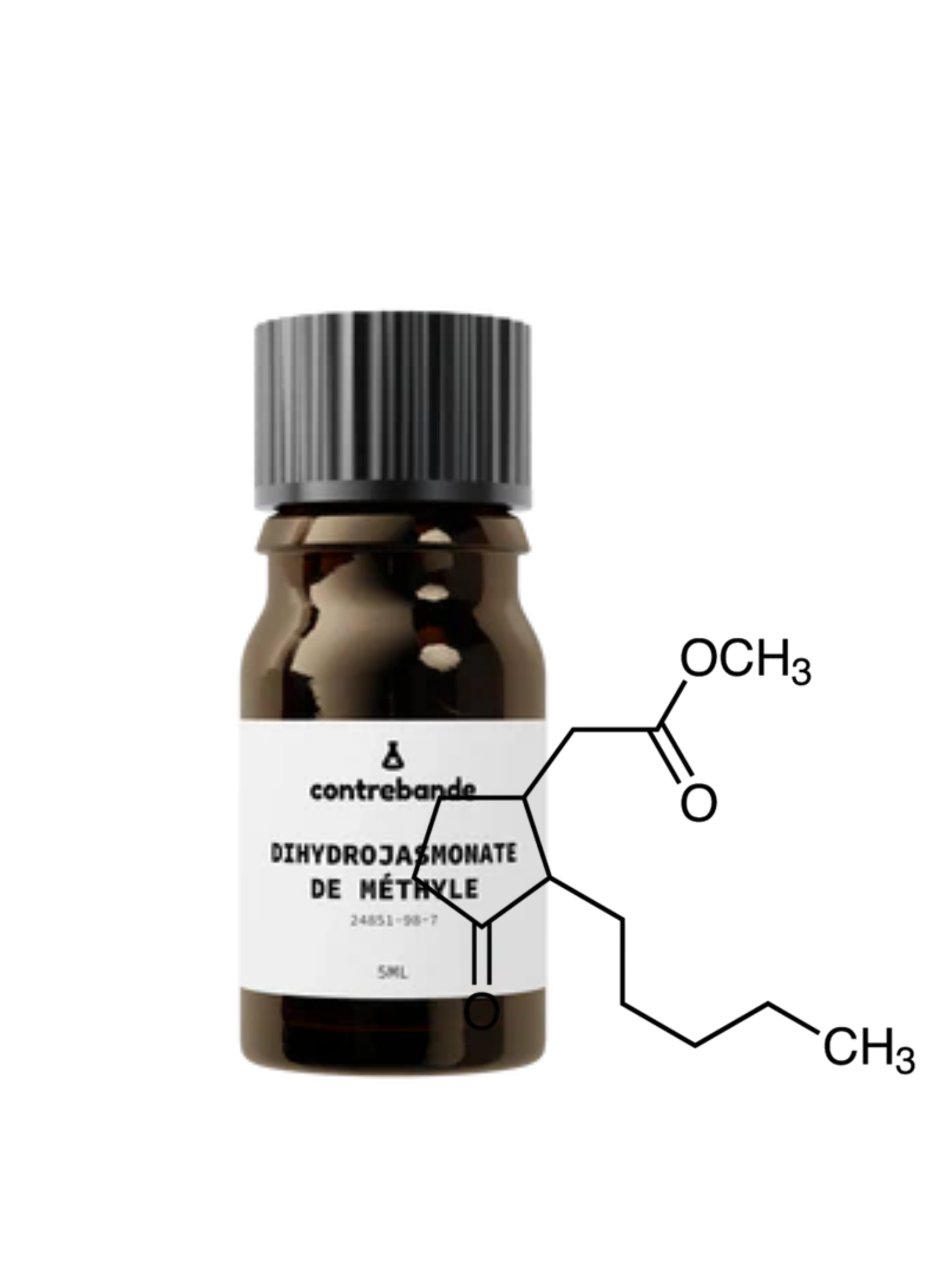
This document provides a comprehensive summary of the human health toxicological data currently available pertaining to the safety evaluation of methyl dihydrojasmonate (see Fig. 1) when used as a fragrance ingredient.
All safety data on methyl dihydrojasmonate were compiled with due diligence including published and unpublished data. In 2008, a complete literature search was conducted on methyl dihydrojasmonate. On-line toxicological databases were searched including those from the Chemical Abstract Services (e.g. ToxCenter (which in itself contains 18 databases including Chemical Abstracts)), and the National Library of Medicine (e.g. Medline, Toxnet (which contains 14 databases)) as well as 26 additional sources (e.g. BIOSIS, Embase, RTECS, OSHA, ESIS). In addition, all RIFM sponsored studies and studies from fragrance companies are included in this summary.
The safety data on this material were last reviewed by Ford (1992). Data from all relevant references are summarized in this FMR. Commonly used terms and their abbreviations are listed in Appendix A. References that are not included are listed in Appendix B. More details have been provided for unpublished data. The number of animals, sex and strain are always provided unless they are not given in the original report or paper.
Papers in which the vehicles and/or the doses are not given were included and noted in this summary because either they demonstrated an adverse effect or there were limited to no data on this fragrance ingredient.
3. Section snippets
1. Identification
2.1.
Synonyms: 2-Amylcyclopentanoneacetic acid, methyl ester; Cyclopentaneacetic acid, 3-oxo-2-pentyl-, methyl ester; Dihyrojasmonic acid methyl ester; Hedione; Methydihydro-Jasmonate; Methyl (2-amyl-3-oxocyclopentyl)acetate; Methyldihydrojasmonate; Methyl 3-oxo-2-pentylcyclopentaneacetate; Methyl (3-oxo-2-pentylcyclopentyl)acetate; Methyl (2-pentyl-3-oxocyclopentyl)acetate; 3-oxo-2-pentylcyclopentaneacetic acid, methyl ester…
- 2.
CAS Registry Number: 24851-98-7
- 3.
EINECS Number: 246-495-9
- 4.
Formula: C13H22O3
- 5.
2. Physical properties
3.1.
Physical form: Pale yellowish or almost colorless oily liquid with a warm, sweet-floral, jasmine-like and fruity odor of moderate to good tenacity
- 2.
Boiling point: 309 °C
- 3.
Henry’s law (calculated): 0.000000502 atm m3/mol @ 25 °C
- 4.
Log Kow (calculated): 2.98
- 5.
Log Kow (measured): 3.1 @ 35 °C
- 6.
Refractive index: 1.457–1.462 @ 20 °C
- 7.
Specific gravity: 0.998–1.006 @ 20 °C
- 8.
Vapor pressure (calculated): <0.001 @ 20 °C
- 9.
Water solubility (calculated): 91.72 mg/l @ 25 °C
4. Usage
Methyl dihydrojasmonate is a fragrance ingredient used in many fragrance mixtures. It may be found in fragrances used in decorative cosmetics, fine fragrances, shampoos, toilet soaps and other toiletries as well as in non-cosmetic products such as household cleaners and detergents. It is a pale yellowish or almost colorless oily liquid with a warm, sweet-floral, jasmine-like and fruity odor of moderate to good tenacity (Arctander, 1969). This material has been reported to occur in nature, with…
5. Conflict of Interest
Joseph Scognamiglio, Leah Jones, Charlene Letizia, and Anne Marie Api are employees of the Research Institute for Fragrance Materials, an independent research institute supported by the manufacturers of fragrances and consumer products containing fragrances.
3. Methyl Jasmonate: Behavioral and Molecular Implications in Neurological Disorders
Oritoke Modupe Aluko1,2,3, Joy Dubem Iroegbu2, Omamuyovwi Meashack Ijomone2,4, Solomon Umukoro3
1Department of Physiology, 2The Neuro-Lab, School of Health and Health Technology, Federal University of Technology, Akure, 3Department of Pharmacology and Therapeutics, University of Ibadan, Ibadan, 4Department of Human Anatomy, School of Health and Health Technology, Federal University of Technology, Akure, Nigeria
Correspondence to: Oritoke Modupe Aluko
Department of Physiology, School of Health and Health Technology, Federal University of Technology, Akure 340252, Ondo, Nigeria
E-mail: omaluko@futa.edu.ng
ORCID: https://orcid.org/0000-0002-6385-8229
1. Abstract
Methyl jasmonate (MJ) is a derivative of the jasmonate family which is found in most tropical regions of the world and present in many fruits and vegetables such as grapevines, tomato, rice, and sugarcane. MJ is a cyclopentanone phytohormone that plays a vital role in defense against stress and pathogens in plants.
This has led to its isolation from plants for studies in animals. Many of these studies have been carried out to evaluate its therapeutic effects on behavioral and neurochemical functions. It has however been proposed to have beneficial potential over a wide range of neurological disorders. Hence, this review aims to provide an overview of the neuroprotective properties of MJ and its probable mechanisms of ameliorating neurological disorders.
The information used for this review was sourced from research articles and scientific databases using ‘methyl jasmonate’, ‘behavior’, ‘neuroprotection’, ‘neurodegenerative diseases’, and ‘mechanisms’ as search words. The review highlights its influences on behavioral patterns of anxiety, aggression, depression, memory, psychotic, and stress.
The molecular mechanisms such as modulation of the antioxidant defense, inflammatory biomarkers, neurotransmitter regulation, and neuronal regeneration, underlying its actions in managing neurodegenerative diseases like Alzheimer’s and Parkinson’s diseases are also discussed.
2. INTRODUCTION
Jasmonates are cyclopentanone phytohormones that play an imperative role in the defense of plants against abiotic stressors and pathogenic invasions [1]. Although they were initially isolated from Jasminum grandiflorum L., a plant mostly found in tropical regions [2], they are extensively distributed in plants and some microorganisms [1].
They are cell regulators, known to activate intracellular signaling mechanisms in plant growth, defense, and response to stress triggers [3]. Their biosynthesis from linolenic acid in plants is analogous to the synthesis of eicosanoids from arachidonic acid in animals [3,4]. The family of jasmonates includes Cis-jasmone (CJ), Jasmonic acid (JA), and Methyl jasmonate (MJ) [3].
Of all the members of the Jasmonates family, MJ is the most studied. MJ is an adaptogenic phytohormone [5] released by plant cells in response to environmental stress, injury, and pathogen invasions. It induces the synthesis of proteinase inhibitor proteins, which are involved in plants’ defense against a variety of biotic and abiotic stressors [5]. On exposure of plants to stressors, MJ is synthesized, resulting in the activation of the proteinase inhibitor gene and subsequently, the expression of proteinase inhibitor proteins [5,6].
Its involvement in the adaptation of plants to stress is further supported by its increased level following plants’ exposure to stressors [5,7]. It also plays a vital role in intracellular signaling and defense in response to pathogenic invasions [1]. One of the numerous adaptogenic properties of MJ relies on its ability to regulate the activities of antioxidants and combat the harmful effects of oxidant molecules [8].
3. SAFETY AND TOXICITY
The ability of MJ to offer cellular protection has generated more attention for its potential use as a therapeutic agent in various disorders and diseases. This has led to the screening of MJ for potential toxicity by several authors [1,2,7,10,12,24,25]. In an investigation by Flescher [12], and Cohen and Flescher [1], MJ administration preferentially killed cancer cells, without affecting normal body cells. Umukoro and Olugbemide [2] also reported no case of toxicity or death in mice after administering 100−500 mg/kg of MJ.
However, results of several studies on acute toxicity, skin irritation, mucous membrane (eye) irritation, skin sensitization, phototoxicity, and photoallergy of MJ indicated that the LD50 for oral administration was > 5 g/kg, and for skin use, the LD50 was > 2 g/kg. Additionally, no irritation was observed in the human repeated-insult patch test and several animal studies. Furthermore, no irritation was detected in the mucous membrane test.
Sensitization reactions in animal and human studies and photo-irritation and photoallergy studies in humans did not show any significant toxicity [25]. This finding further support previous investigations, which show that MJ is safe, as it is not toxic to normal body cells [1,7]. Likewise, the US Federal Environmental Protection Agency in 2013 issued MJ an exclusion for tolerance requirement test as it was observed to be naturally-available in human nutrition [26].
4. METHYL JASMONATE MODIFIES BEHAVIORS ASSOCIATED WITH NEUROLOGICAL DISORDERS
1. Anxiety/Anxiolytic Activity
Anxiety is a disorder of the central nervous system (CNS) associated with an imbalance between excitatory and inhibitory impulses in the brain. These imbalance areas result in decreased GABAergic and increased glutaminergic neurochemical pathways respectively [28-32]. Anxiety manifests in various ways like fear, eating disorder, worry, suicidal tendencies in humans [22]. Several studies have explored the anti-anxiolytic potential of MJ.
Most of which used mice models. Umukoro et al. [22] demonstrated the anti-anxiolytic effect of MJ on unpredictable chronic mild stress (UCMS)-induced mice while studying the explorative behavior of the mice in a light/dark transition test and elevated plus maze (EPM) test. In the EPM test, MJ reduced the frequency and extent of time spent in the closed arm in UCMS-induced mice. MJ also reduced the time spent by mice in the dark compartment in a light/dark transition test of UCMS-induced mice. All these observations suggest the anti-anxiogenic activity of MJ [22].
2. Depression
Depression is a prevalent disorder that negatively impacts the quality of life worldwide. It affects about 20% of the world’s population and is typically higher in females than in males with a ratio of 5:2. Preclinical and clinical investigations have implicated serotonin and norepine-phrine in its pathogenesis [33,34].
The deficiency of these monoaminergic transmitters in the brain is reported to be one of the most significant etiological factors for the cause of depression. The recurrent nature of depression and its numerous triggers have made it difficult to manage [34-36]. These have led to increased interest in researching more effective antidepressants [37,38]. In a study conducted by Adebesin et al. [18], an acute stress model of tail suspension test (TST) was adapted to investigate the antidepressant-like property of MJ in UCMS-induced mice.
An increased latency period was observed in UCMS-induced mice. This period was significantly reduced, following treatment with MJ, indicating antidepressant-like property [18].
This finding is consistent with that of Umukoro et al. [37] where acute stress models of TST and forced swim test (FST) were adapted in mice to study the antidepressant activity of MJ. MJ significantly decreased the period of immobility in both tests. Adebesin et al. [18] went further by using the sucrose preference test to evaluate the anti-depressant activity of MJ. This test is used to evaluate anhedonia (inability to experience pleasure), a key symptom of depression in humans.
They reported that MJ attenuated impaired sucrose intake in rodents initially exposed to UCMS [18]. Biochemical evaluations have also been carried out to confirm the anti-depressant property of MJ. In a study by Zomkowski et al. [39], MJ reduced serotonin levels [39]. Studies have shown also that the anti-immobility exhibited by antidepressants in the FST and TST is mediated through the facilitation of both serotonergic and noradrenergic neurotransmissions [37.
3. Aggression
Aggression is a deliberate series of actions that inflict harm on another organism and is a major component of the stress-syndrome. It is characterized by low tolerance to frustration and studies have shown that feeling of frustration results from prolonged stress [40,41]. Aggression may manifest itself as a defensive or offensive behavior. Although aggression and depression are diagnostically categorized differently by the psychiatric classification systems Diagnostic and Statistical Manual of Mental Disorders 4th edition, they are however clinically and biochemically related [35,37].
The serotonergic system is implicated in both disorders [35]. This is proven by alleviated symptoms of depression and aggression when serotonin receptor agonists and uptake inhibitors were administered [35]. In a study by Umukoro et al. [42], MJ (1, 5, 10 mg/kg, intraperitoneally [i.p.]) had a dose-dependent decrease in aggressive behaviors in resident-intruder and isolation-evoked paradigms (both measures offensive aggression) in mice.
Although MJ has an anti-aggressive activity, it, however, does not impair the defense mechanism of the animals. These findings suggest the therapeutic usefulness of MJ as an anti-aggressive agent. Its ability to maintain the defense mechanism in animals suggests that it could be a better therapeutic approach to aggressive behaviors than antipsychotics and high doses of benzodiazepines which tends to impair the defensive mechanisms of organisms [43].
Of all the neurochemicals associated with aggressive behaviors, reduced 5-HT has been recurrently linked with aggression by numerous authors [43,44].
4. Memory/Cognitive Enhancement
MJ is used extensively in aromatherapy as a therapeutic agent for memory dysfunction [9]. In a study conducted by Umukoro et al. [22], intraperitoneal injection of MJ (25, 50, and 100 mg/kg) improved memory performance in mice exposed to UCMS. MJ was further shown to reverse UCMS-induced neurodegeneration in the sub-granular zone of the dentate gyrus and the pyramidal layer of the CA3 [22].
These learning and memory associated regions of the brain have been reported to exhibit loss of dendritic spines [47] and a reduced number of synapses [48] following UCMS. The results of the study established that UCMS produced the death of neuronal cells in the pyramidal layer of the CA3 and the sub-granular zone of the dentate gyrus of the hippocampus, the regions of the brain that plays vital roles in learning and memory [22]. Thus, a decrease in hippocampal density may lead to loss of memory function [49].
Previous clinical studies have linked reduced hippocampal volume to memory and cognitive impairment in patients with Alzheimer’s disease (AD) [49,50]. Thus, oxidative stress-mediated hippocampal neuronal degeneration highlights memory impairment due to chronic stress. However, there are suggestions that compounds with a neuroprotective property may be of benefits in chronic stress-induced cognitive deficits and other neuropsychiatric disorders [51,52].
In another study, Eduviere et al. [19] used the passive avoidance paradigm to evaluate the influence of MJ on rat memory. This model uses aversive stimuli associated with fear as a condition for learning and memory acquisition [53,54]. This model assesses both the role of the hippocampus in memory [55] and the amygdala in fear-conditioned learning and memory [56].
5. Antipsychotic
Psychosis is a form of mental illness characterized by abnormal behaviors with little or no touch with reality [58]. It is characterized by multiple symptoms affecting thoughts, emotion, perception, and volition. It is a severe form of mental illness affecting the quality of life of the affected individuals [17]. Although pharmacological interventions have been the backbone of treatment of the disease, the use of antipsychotic drugs has certain limitations.
These include the incidences of poor adherence, limited responses, and other incapacitating outcomes [59]. More notably, these drugs have failed to alter the course of the disease but are known to only provide symptomatic relief [17]. Likewise, the associated negative symptoms and memory deficits are not relieved by the antipsychotics [60-62].
Thus, the need to search for new drugs, especially agents with potential memory-enhancing effects as alternative treatments for psychotic disorders. Annafi and colleagues [17] adapted the bromocriptine-induced and ketamine-induced stereotypes as models to screen for the antipsychotic-like effect of MJ
6. Anti-stress
Increasing the prevalence of physical, biological, or psychological stressors lead to an increase in stress and subsequently a rise in dyshomeostasis [63,64]. Organisms normally respond to acute stress by adapting to the changes in their environment. However, prolonged stress leads to illness or cell damage. Prolonged stress has been implicated in a variety of diseases such as hypertension, immune dysfunction, cancer, and several neurodegenerative disorders [23,64]. Adaptogens are a classified group of substances with the ability to improve the mental and physical performances of organisms during exposure to stressful stimuli [65]. Numerous studies have employed behavioral, and biochemical techniques to demonstrate the anti-stress property of MJ [22,23].
MJ decreased the immobility time in FST and increased the latency to convulsion in the hypoxia test in mice exposed to acute stress [23]. MJ was shown to reduce the level of corticosterone secretion in stressed mice indicating its adaptogenic-like property. Corticosterone induces brain damage by increasing the intracellular level of oxidative stress. Chronic stress is known to trigger corticosterone release via the hypothalamic-pituitary-adrenal axis. This finding is further backed up by an increase in the adrenal gland and liver size which was noticed in UCMS-induced rats. Increased corticosterone levels can cause further damage via oxidative stress [50,66] and neuroinflammation [67].
Question
- Is hedione natural?
From the Greek word ‘hedone’ which means pleasant, we can safely say it most certainly is. Synthesised in 1962, hedione is in fact present in natural Jasmine extracts and is frequently used to recreate jasmine notes in fragrances.
2. What are the benefits of hedione?
Hedione enhances a fragrance by increasing its diffusion and making its scent more noticeable. It achieves this by interacting uniquely with the olfactory receptors and the limbic system—the part of the brain responsible for emotions and memories.20-May-2024
3. Is methanol good for perfume?
Methanol is a different alcohol entirely than ethanol. It’s extremely toxic and readily absorbed by the skin. You should never even consider using methanol in perfumes.03-Aug-2022
4. What is methanol medicine used for?
Methanol is primarily used as an industrial solvent to help create inks, resins, adhesives, and dyes. It is also used as a solvent in the manufacture of important pharmaceutical ingredients and products such as cholesterol, streptomycin, vitamins and hormones.
5. What are the effects of Hedione?
Hedione’s Impact on the Brain
When compared with the traditional floral fragrance phenylethyl alcohol, Hedione activated brain areas in the limbic system more strongly. This part of the brain is associated with emotions, memory, and motivat.